

 |
 |
| Antimony | |
| Electron Configuration | 2,8,18,18,5 |
| Electron Configuration | [Kr] 4d10 5s2 5p3 |
| Ground state | 4S°3/2 |
| Atomic Volume | 18.23 g/cm3 |
| Electronegativity | 2.05 |
| Magnetic ordering | No data |
| Mass magnetic susceptibility | -1.09 x 10-8 |
| Molar magnetic susceptibility | -1.327 x 10-9 |
| Speed of sound | 3420 m s-1 |
 Thermal Properties Thermal Properties |
|
| Enthalpy of Atomization | 263.6 kJ mol-1 @25°C |
| Enthalpy of Fusion | 20.9 kJ mol-1 |
| Enthalpy of Vaporisation | 165.8 kJ mol-1 |
| Heat Capacity | 25.23 J mol-1 K-1 |
| Thermal Conductivity | 24.4 W m-1 K-1 |
| Thermal Expansion | 11 μm m-1 K-1 |
 Hardness Hardness |
|
| Brinell hardness | 294 MPa |
| Mohs hardness | 3.0 |
 Elastic Properties Elastic Properties |
|
| Bulk modulus | 42 GPa |
| Shear modulus | 20 GPa |
| Young's modulus | 55 GPa |
 Electrical Properties Electrical Properties |
|
| Electrical resistivity | 4.0 x 10-7 Ω m |
| Electrical conductivity | No data. |
 Chemical Properties Chemical Properties |
|
| Electrochemical Equivalent | 1.5142 g Ah-1 |
| Electron Work Function | 4.55 eV |
| Valence Electron Potential (-eV) | 57 |
| Ionization Potential | |
| 8.641 | |
| 16.53 | |
| 25.3 | |
| Incompatibilities | Strong oxidizers, acids, halogenated acids. |
 Flammability Flammability |
|
 Energy Levels Energy Levels |
|
| 26.3591 KeV | |
| 26.1108 KeV | |
| 29.7256 KeV | |
| 30.3844 KeV | |
| 29.6743 KeV | |
| 3.60472 KeV | |
| 3.59532 KeV | |
| 3.84357 KeV | |
| 4.10078 KeV | |
| 3.9311 KeV | |
| 3.8853 KeV | |
| 4.34779 KeV | |
| 4.5977 KeV | |
| 3.435 KeV | |
| 3.1868 KeV | |
 Atomic Radii Atomic Radii |
|
| 145 pm | |
| 133 pm | |
| 138 pm | |
| No data. | |
| 120 pm | |
| No data. | |
 Ionic Radii (Å) Ionic Radii (Å) |
||||
| Charge | Coordination | Crystal | Ionic | Key |
| 3 | IVPY | 0.9 | 0.76 | |
| V | 0.94 | 0.8 | ||
| VI | 0.9 | 0.76 | A | |
| 5 | VI | 0.74 | 0.6 | * |
| R, From r3 vs V plots. |
| C, Calculated from bond length - bond strength equations. |
| E, Estimated. |
| *, Most Reliable. |
| M, From Metallic Oxides. |
| A, Ahrens (1952) Ionic radius. |
| P, Pauling's (1960) Crystal Radius. |
 Oxidation States Oxidation States |
|
| Sb+3, Sb+5 | |
| Sb-3 | |
 Ionisation Energies (kJ mol-1) Ionisation Energies (kJ mol-1)  |
|
| 833.7 | |
| 1794 | |
| 2443 | |
| 4260 | |
| 5400 | |
| 10400 | |
| 12700 | |
| 15200 | |
| 17800 | |
| 20400 | |
 Covalent Bonds (kJ mol-1) Covalent Bonds (kJ mol-1) |
|
| 257 | |
| 215 | |
| 314 | |
| 389 | |
| 313 | |
| 299 | |
 Vapour Pressure Vapour Pressure |
||||||
| P (Pa) | 1 | 10 | 100 | 1K | 10K | 100K |
| T (K) | 807 | 876 | 1011 | 1219 | 1491 | 1858 |
 Crystal Structure Crystal Structure |
|
| Trigonal | |
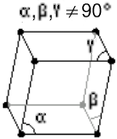 |
a = 430.7 pm |
| b = 430.7 pm | |
| c = 1127.3 pm | |
| α = 90° | |
| β = 90° | |
| γ = 120° | |