

 |
 |
| Neon | |
| Electron Configuration | 2,8 |
| Electron Configuration | 1s2 2s2 2p6 |
| Ground state | 1S0 |
| Atomic Volume | 16.7 g/cm3 |
| Electronegativity | No data. |
| Magnetic ordering | No data |
| Mass magnetic susceptibility | -4.1 x 10-9 |
| Molar magnetic susceptibility | -8.27 x 10-11 |
| Speed of sound | 435 m s-1 |
 Thermal Properties Thermal Properties |
|
| Enthalpy of Atomization | No data. |
| Enthalpy of Fusion | 0.324 kJ mol-1 |
| Enthalpy of Vaporisation | 1.736 kJ mol-1 |
| Heat Capacity | 20.786 J mol-1 K-1 |
| Thermal Conductivity | 49.1 W m-1 K-1 |
| Thermal Expansion | No data. |
 Electrical Properties Electrical Properties |
|
| Electrical resistivity | No data. |
| Electrical conductivity | No data. |
 Chemical Properties Chemical Properties |
|
| Ionization Potential | |
| 21.564 | |
| 40.962 | |
| 63.45 | |
 Optical Properties Optical Properties |
|
| Refractive Index (gas) | 1.000067 |
 Energy Levels Energy Levels |
|
| 0.8486 KeV | |
| 0.8486 KeV | |
| 0.857 KeV | |
 Atomic Radii Atomic Radii |
|
| 51 pm | |
| 38 pm | |
| 69 pm | |
| 154 pm | |
| No data. | |
| No data. | |
 Ionisation Energies (kJ mol-1) Ionisation Energies (kJ mol-1)  |
|
| 2080.6 | |
| 3952.2 | |
| 6122 | |
| 9370 | |
| 12177 | |
| 15238 | |
| 19998 | |
| 23069 | |
| 115377 | |
| 131429 | |
 Vapour Pressure Vapour Pressure |
||||||
| P (Pa) | 1 | 10 | 100 | 1K | 10K | 100K |
| T (K) | 12 | 13 | 15 | 18 | 21 | 27 |
 Crystal Structure Crystal Structure |
|
| Cubic close packed | |
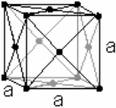 |
a = 442.9 pm |
| b = 442.9 pm | |
| c = 442.9 pm | |
| α = 90° | |
| β = 90° | |
| γ = 90° | |